Autoimmune Diseases and Inflammation
The immune system protects the body from disease and infection. In people with autoimmune disorders, though, the immune system can attack healthy body cells by mistake. There are more than 80 types of autoimmune diseases, according to the National Library of Medicine. Autoimmune diseases include rheumatoid arthritis, psoriasis, inflammatory bowel disease, lupus, Sjogren’s disease, multiple sclerosis, Hashimoto’s disease, Celiac disease and type 1 diabetes.
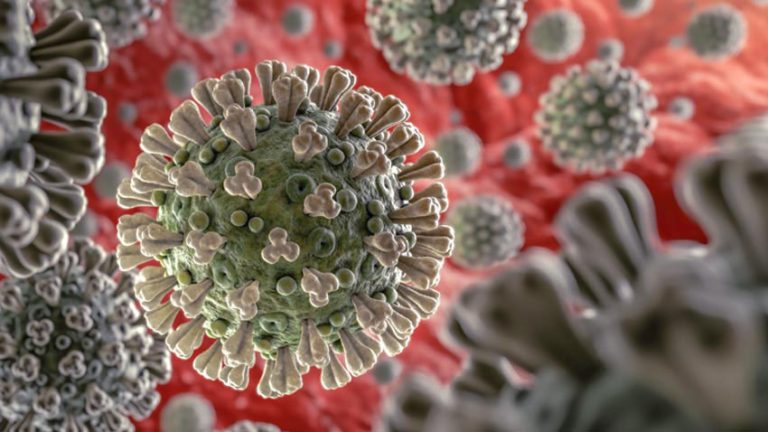
COVID-19 and Inflammation
The virus, known as SARS-CoV-2 or simply “coronavirus,” causes a wide variety of symptoms associated with COVID-19 disease. Most notably, the virus causes cough, fever, and shortness of breath. Coronavirus is highly contagious, already infecting at least 2 million people in the United States by the middle of June 2020, and claiming the lives of 113,000. While people with autoimmune disorders are not more likely to contract coronavirus than are the rest of the general population, they are more likely to develop severe complications if they do contract COVID-19 if they have a suppressed immune system due to their autoimmune disease or treatment for their autoimmune disorder. One of the most serious complications of COVID-19 is severe inflammation throughout the body, including the lungs, heart and brain. The body reacts to the presence of the SARS-CoV-2 virus with a robust inflammatory response; health professionals now regard this excessive inflammatory response as a hallmark symptom of COVID-19.
How Autoimmune Diseases Cause Inflammation
Special receptors cover the exterior surface of body cells. Proteins bind to these receptors to change the way the cell works. A specific type of protein, known as cytokines, binds to certain receptors to regulate the body’s immune response. Cytokines are mediators, which mean they trigger and control a body response. Specifically, cytokines mediate the inflammation response to tissue injury or infection. In other words, cytokines promote inflammation as a response to tissue injury or infection. There are several types of cytokines, and each type can work alone, work together, or work against each other to regulate the immune response. A special type of cytokine, known as interleukin or IL, may play an important role in the immune response in COVID-19 patients. There are 40 interleukins, IL-1 through IL-40, and each performs a function. Interleukins normally help the immune system fight off viruses and bacteria in the body, but an overactive immune system can cause interleukins to attack the body instead. This can lead to chronic inflammatory conditions.
A proprietary state-of-the art data mining bioinformatics program, called ‘cluster analysis’ will be used to measure disease development susceptibility with potential for earlier diagnosis and intervention. The company is developing a healthcare program based on its proprietary genetic panels that will allow people to be their own healthcare advocate and take an active role in their health status as well as longevity.


SOURCES:
https://medlineplus.gov/autoimmunediseases.htmlhttps://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.htmlhttps://www.ncbi.nlm.nih.gov/books/NBK499840/https://www.medscape.com/answers/2500114-197455/what-is-the-role-of-interleukin-il-inhibitors-in-the-treatment-of-coronavirus-disease-2019-covid-19