Lyme Disease Trends: Northeast
 State health departments report about 30,000 cases of Lyme disease each year, according to the Centers for Disease Control and Prevention (CDC), but that number does not accurately reflect the actual number of cases diagnosed around the United States. Some studies suggest the actual number of Lyme disease could be as high as 444,000.
State health departments report about 30,000 cases of Lyme disease each year, according to the Centers for Disease Control and Prevention (CDC), but that number does not accurately reflect the actual number of cases diagnosed around the United States. Some studies suggest the actual number of Lyme disease could be as high as 444,000.
About Lyme Disease and its Transmission
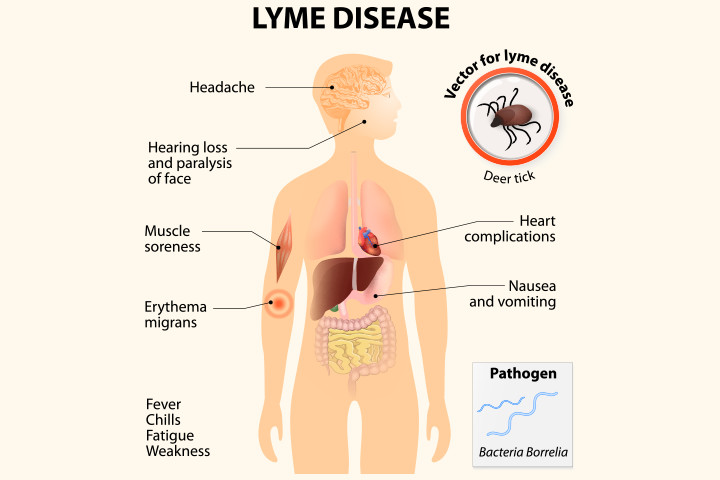
Lyme disease is a vector-borne disease, which means a carrier moves from one host to another. This carrier, known as a vector, transmits the illness but the vector does not become infected with the disease.
The deer tick is the vector for Borrelia burgdorferi, the bacterium responsible for Lyme disease. Transmission occurs when a tick carrying the Borrelia burgdorferi bites a human to feed on the individual’s blood. The bacterium moves from the intestines of the tick through its mouthparts and into the human’s bloodstream while the tick feeds.
While a number of mammals carry Borrelia burgdorferi bacterium in their blood, the most common source of infection is the white-footed mouse.
Deer ticks are most likely to transmit the bacteria after remaining attached and feeding for two or more days. Ticks are most active from April to October in most areas but may bite year-round in milder climates.
Infection occurs quickly. Research shows traces of Borrelia burgdorferi can appear in the nervous system only 12 hours after infection. Lyme disease causes a rash, usually in a bull’s-eye pattern, and flu-like symptoms. Joint pain, headaches, and weakness in the limbs can also occur.
Doctors typically treat Lyme disease with antibiotics. Patients usually enjoy a full recovery, although some patients experience symptoms that linger for six months or longer, a condition known as Post-Treatment Lyme Disease Syndrome (PTLDS) or chronic Lyme disease. Pain medicine can help those with PTLDS overcome lingering symptoms.
About Lyme Disease Trends
Lyme disease can affect people of any age or either gender, but it is most common in boys aged 5 to 9 years.
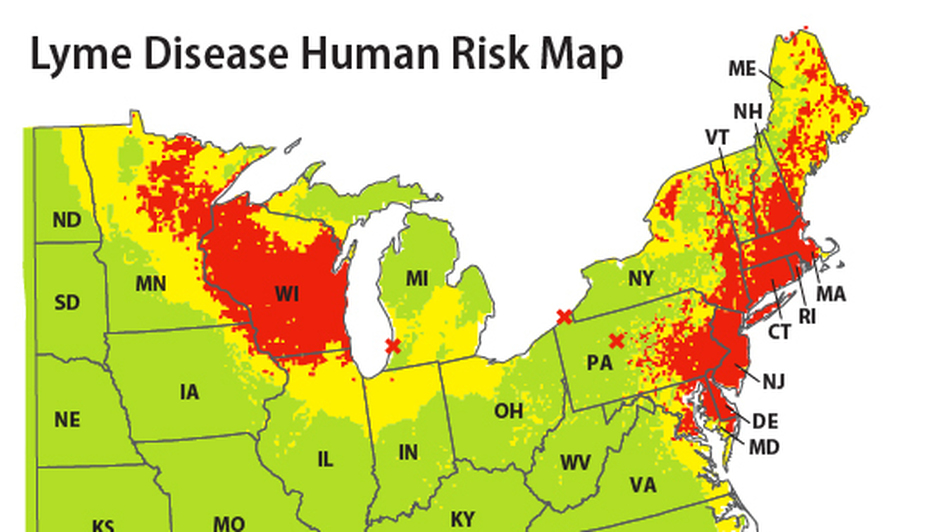
First discovered in Lyme, Connecticut, the disease is still most common in the Midwest and, especially, in Northeastern states. In fact, 14 states account for more than 96 percent of Lyme disease cases reported to the CDC.
The U.S. Environmental Protection Agency (EPA) developed maps that, based on the number of new cases per 100,000 people, illustrate how Lyme disease reports have changed since 1991. Trending statistics include national reports and reports from the 14 states where Lyme disease is most common.
Nationally, there were 3.74 cases of Lyme disease per 100,000 people in 1991 and 7.95 per 100,000 in 2014. The number of cases reached their highest levels in 2013, when there were 8.59 reported cases of the disease per 100,000 people.
New Hampshire, Maine, and Vermont showed the greatest increase in reported cases of Lyme disease at 93.31, 84.13, and 83.02 cases per 100,000, respectively. Extreme year-to-year variations in reporting practices prevented the EPA from calculating trend activity in Connecticut, New York, and Rhode Island.
For more information on Lyme disease, consult with your doctor or another healthcare professional.
Source
http://www.cdc.gov/lyme/stats/humancases.html
http://www.caryinstitute.org/science-program/research-projects/lyme-disease
https://www.epa.gov/climate-indicators/climate-change-indicators-lyme-disease
https://www.epa.gov/sites/production/files/2016-08/lyme_fig-2.csv
Frank Magliochetti is Managing Partner for Parcae Capital.
-
North Andover, Massachusetts
This column of posts is directed at the Healthcare Industry. Frank plans to release a new site dedicated to the industry. He currently assists companies who are building, restructuring, transforming and resurrecting there business’s. An example of his client base are, Xenetic Biosciences , IPC Medical Corp, Just Fellowship Corp, Environmental Services Inc., Parsons Post House LLC, ClickStream Corporation as well as having a business talk radio show; The Business Architect on the URBN network.